“The ultimate purpose of life, mind, and human striving: to deploy energy and information to fight back the tide of entropy and carve out refuges of beneficial order.”
Steven Pinker, The Second Law of Thermodynamics (2017)
In the realm of thermodynamics, the term “entropy” represents the measure of disorder or randomness in a system. While the first law of thermodynamics tells us that the total energy of the universe remains constant (the principle of the conservation of energy), this energy becomes progressively less useful as it is used up and spread out. The second law of thermodynamics says that the total disorder (entropy) of the universe increases over time. Energy is not perfectly recyclable; something is always lost.
Entropy may not be intuitive, but its implications ripple across our understanding of the physical world, social systems (such as companies or families), and even the emergence of life on Earth!
Just as a sandcastle will inevitably erode without constant maintenance, so too do the systems in our lives require energy and attention to stave off disorder.
Irreversibility and chaos
A great way to understand why entropy always increases is by exploring a fundamental property of nature: irreversibility. Most processes cannot be perfectly undone. We would struggle to un-cook an egg, to un-mix two combined paint colors, to un-burn firewood, or to un-birth a child.
Imagine applying heat to an ice cube. As the temperature rises, the rigid, frozen water molecules will begin to loosen and scatter as the water goes through phase transitions of melting and eventually vaporizing. Total entropy (disorder) has increased through this process, as some heat (useful energy) was dissipated to cause the phase transitions. We can re-freeze the water vapor by cooling it, but this will require additional energy use, further increasing entropy.[efn_note]Bahcall, S. (2019). Loonshots. St. Martin’s Press. 11-14.[/efn_note]
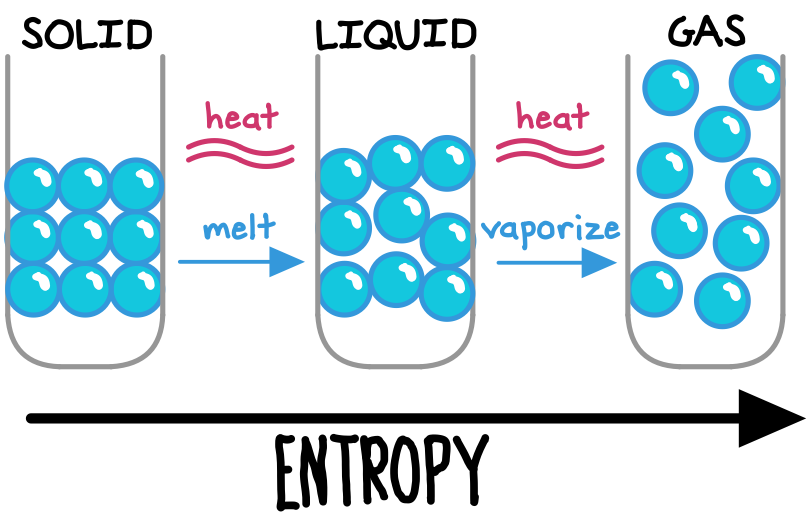
Now imagine we simply place our ice cube on the kitchen counter. The warmer counter will transfer some heat to the ice, until their temperatures equalize. Because the atoms in a hotter substance—by definition—are moving more quickly than those in a colder substance, heat tends overwhelmingly to flow in one direction: from a hotter place to a cooler place. The ice cube does not transfer “coldness” to the counter. Critically, this one-directional property means that we are unable to perfectly “reverse” most heat transfers.[efn_note]Feynman, R., Leighton, R. B., & Sands, M. (2010). The Feynman Lectures on Physics (3rd ed., Vol. I). Basic Books. 44-4.[/efn_note]
Imagine if physical processes—such as melting an ice cube or operating a car engine—were perfectly reversible: we would have infinitely recyclable energy, and there would be no entropy. But in reality, complex natural processes are generally irreversible, meaning that they involve at least some expenditure of useful energy. Consider how even an incredibly efficient car engine requires a non-zero amount of fuel, electricity, or friction to function.
This is thermodynamics’ key limitation: because some useful energy must be expended to do work, no process can be perfectly reversed, and entropy must increase.[efn_note]Feynman, R., et al. (2010). 44-12.[/efn_note]
The improbability of order
Nature’s tendency towards disorder, whether with ice cubes or engines or businesses, can also be understood through probabilities. Processes tend to move from less probable to more probable states. Because the number of ways a system can be ordered is far smaller than the number of ways it can be disordered, disorder is inevitable.
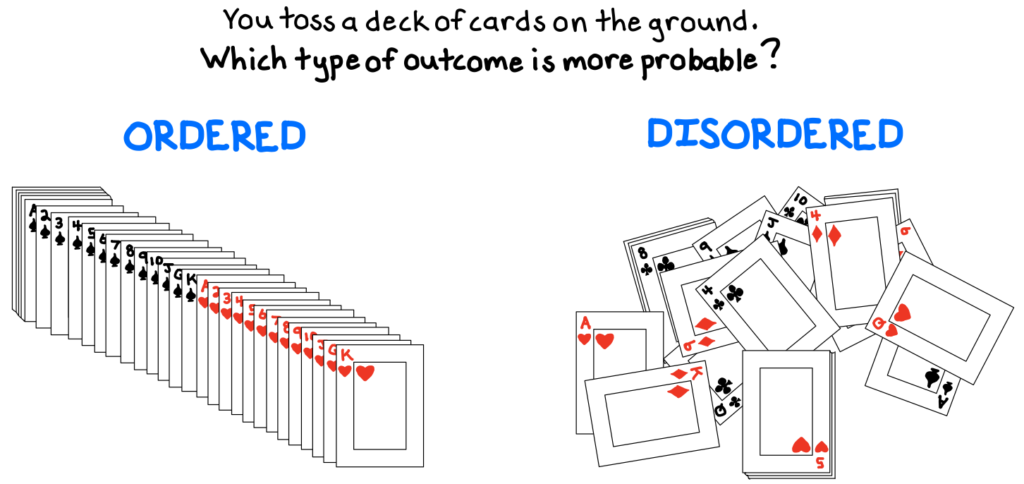
Imagine a deck of cards thrown on the ground. How likely is it that the cards land in an ordered fashion? There are more than 8×1067 ways to arrange a 52-card deck, so the probability of any random arrangement is miniscule. Chaos is much more common. In fact, the change from an ordered arrangement to a disordered one is the source of irreversibility: work must be done in order to re-impose an ordered state on the system![efn_note]Feynman, R., et al. (2010). 46-7.[/efn_note]
Carving out refuges of order
Just as we must do more work to re-freeze our ice cube after it inevitably melts, we must constantly expend effort to create and maintain order in our lives, as time tips them towards chaos and disorder. This includes the condition of our homes, relationships, companies, and physical health.
Bottom line: because disorder is always increasing, complacency will eventually lead to failure.
Though invoking thermodynamical laws in everyday life may not earn us many “cool points,” we can drastically improve our decision making by understanding entropy in the context of social systems. For a social system such as an organization or relationship to be productive, it must be in some useful order, and organizing the people and activities into a useful state requires us to actively invest energy and resources.
In business, companies that don’t work constantly to cultivate and evolve their purpose, structure, and processes, will inevitably stagnate. Over time, bureaucracy and decay will seep in, as the gap widens between the company’s goals and the activities of its employees. Lines of responsibility will blur, and the product portfolio will become bloated. To stave off entropy, management must periodically redesign processes and reallocate resources to ensure the company channels its competitive energy outward, not inward.[efn_note]Rumelt, R. (2011). Good Strategy/Bad Strategy. Crown Business. 214-222.[/efn_note]
Life’s dance with disorder
At a cosmic scale, entropy tells us that all things will fall apart eventually, from our own Sun to the entire Milky Way. This is not something to fear; in fact, the law of entropy can help us appreciate the “miracle” of life on our own planet.
If disorder is always increasing in a closed system, how can it be that highly “ordered” organic life and ecosystems have evolved on Earth? The answer stems from the fact that our planet is not a closed system, but a sub-system within a larger solar system, within a larger galaxy, and so on.
While disorder always increases in the universe at large, order can emerge in smaller systems (such as the Earth) which feed off an outside energy source. Our energy source is the Sun, whose dissipated heat helps create the unique conditions on Earth that enable complex organic systems to emerge.[efn_note]Gribbin, J. (2004). Deep Simplicity. Random House. 26-29.[/efn_note]
In this sense, the universe’s march towards disorder is the very source of the rare, beautiful order of our planet!
***
In conclusion, entropy explains why we are seemingly always battling against forces messing things up in our lives. It also serves as a celebration of the precious and transient order that makes life possible in the first place. There is perhaps no better motivation for us to create and sustain order in the families, communities, and organizations that we cherish.
